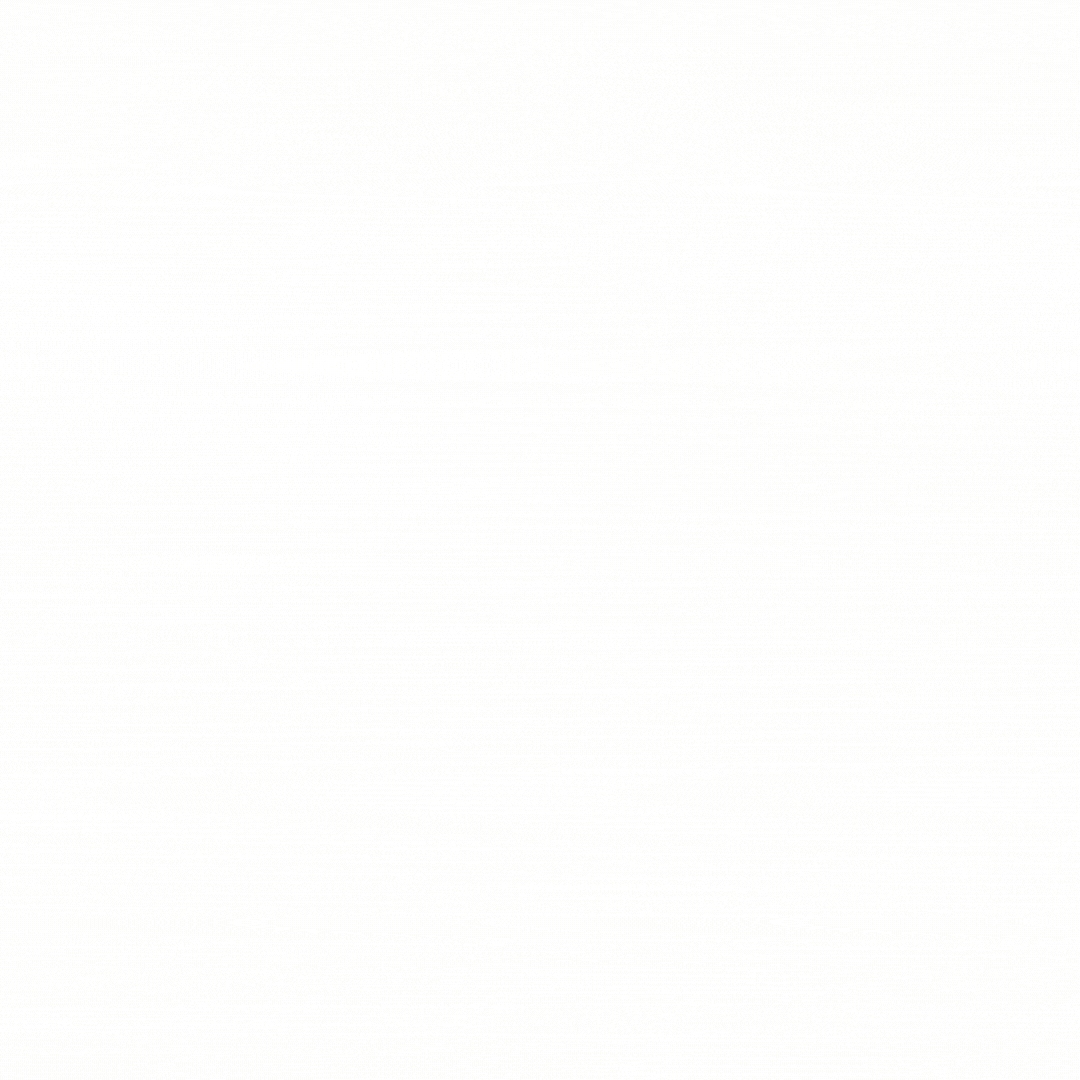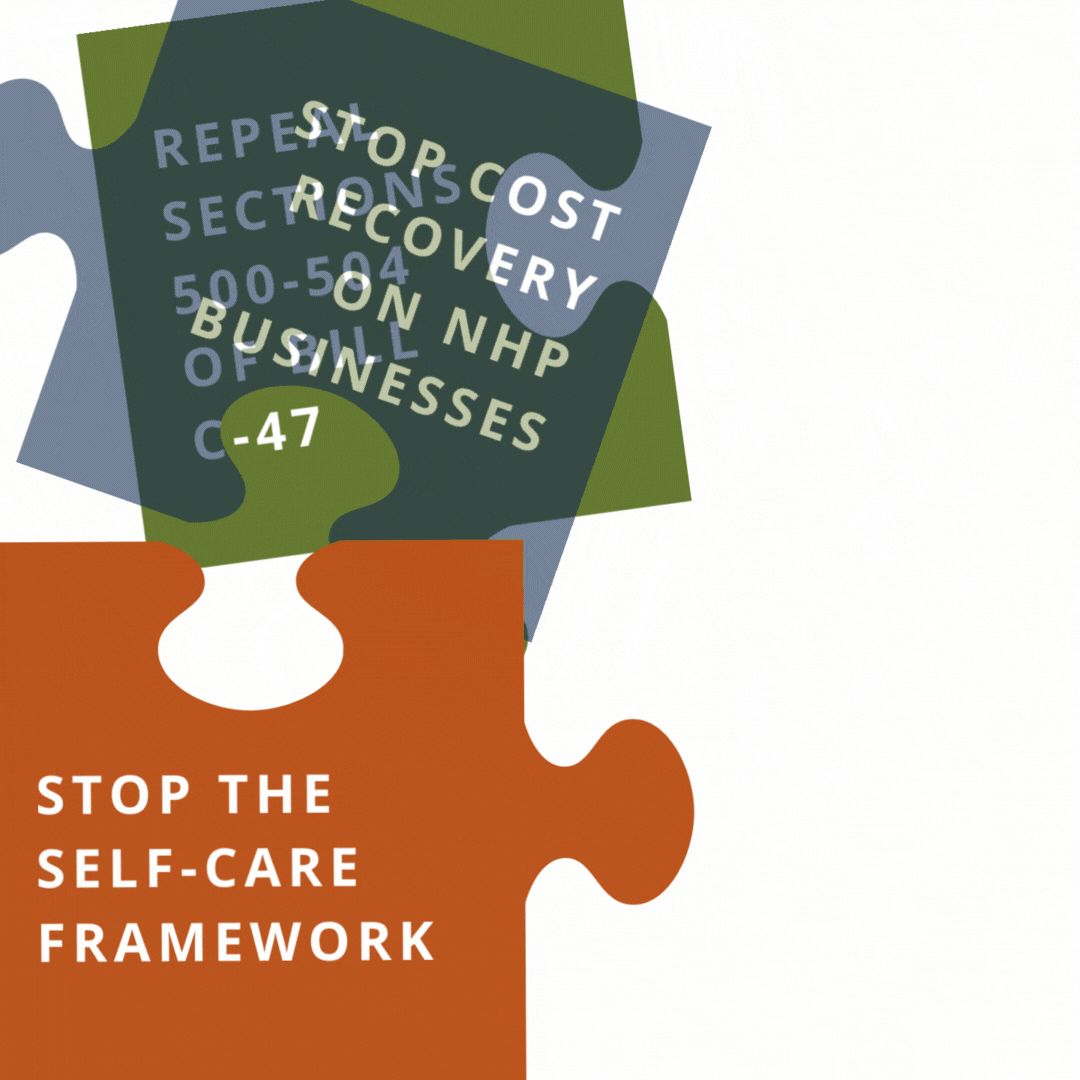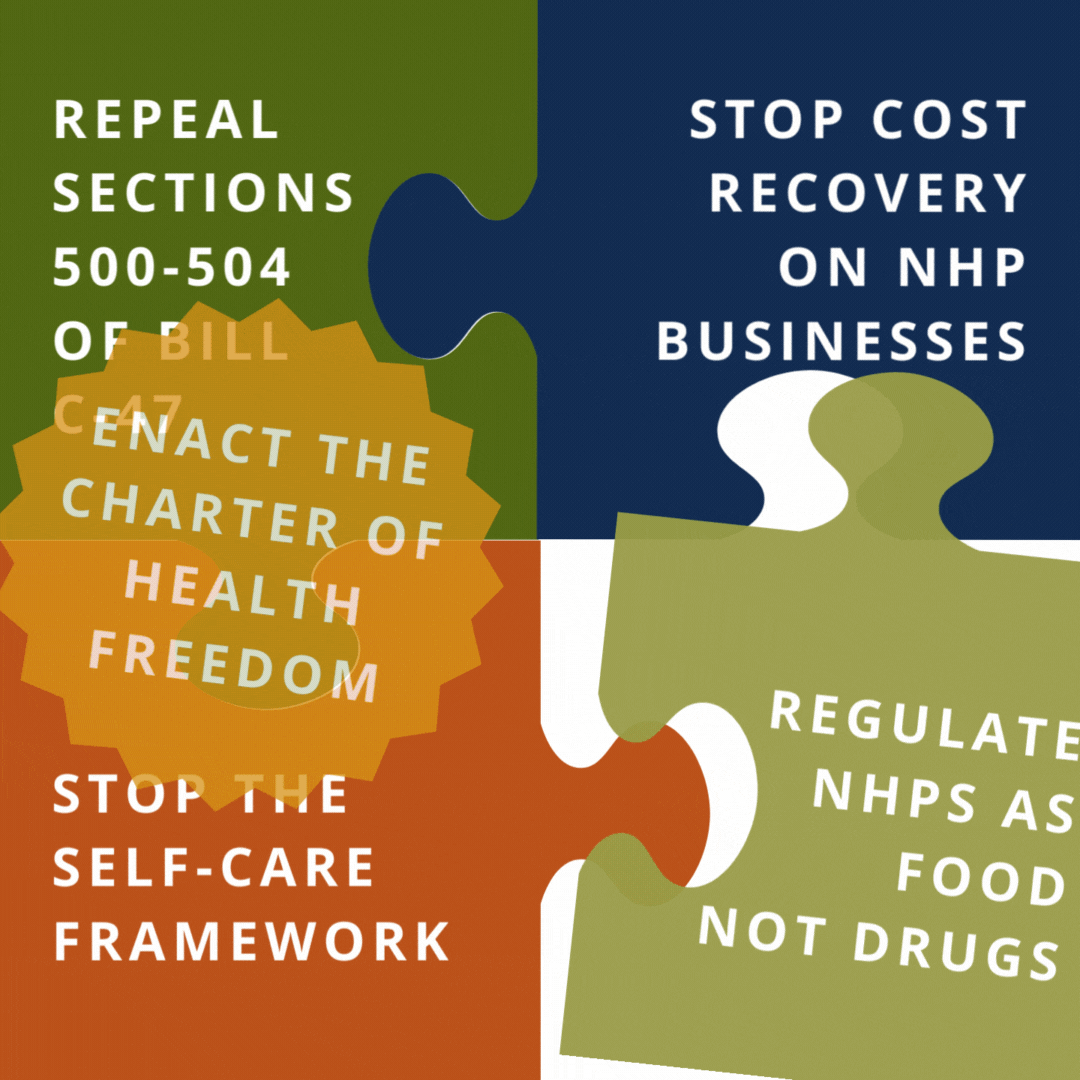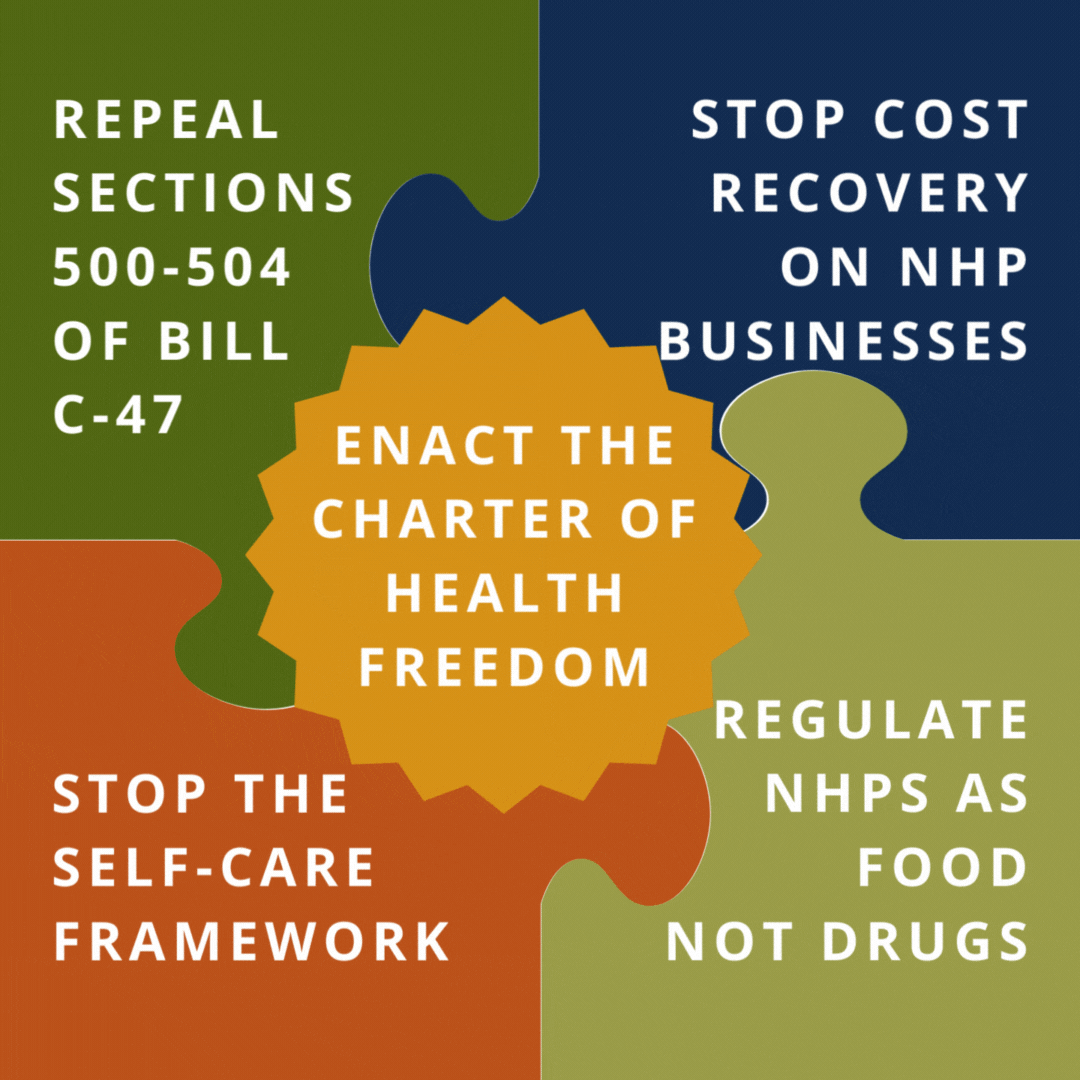Get to know the issues so you can speak and write about them. Watch the 5-minute video above, read our two-page crisis summary, or read our full 35-page Discussion Paper.
Subscribe to our email newsletter for the most up-to-date information and to make sure you never miss an action item.
Help us reach our goal of 250,000 signatures by printing out a Charter of Health Freedom petition, collecting signatures, and mailing it back to us.
You can engage with your MP by writing letters, mailing in our postcards, phoning their office or scheduling an in-person visit.
We rely on your financial support. With every donation, you help ensure that NHPPA can continue to do what we do best.
You can inform others about NHPPA’s work by talking in your community and sharing our content on social media.
We invite you to be part of a nationwide movement to protect Canadians’ access to natural health products.